Sumedh Kothari
Since the 1940s and their invention, PFAS chemicals have been widely used in a variety of technologies, industries, and products that we use today. Recently, there has been a lot of attention regarding their health impacts. But what are they, and are they really dangerous?
Per- and poly-fluoroalkyl substances (PFAS) are chemical surfactants used in many industrial applications and frequently found in daily life products, such as nonstick pans, stain-resistant fabrics, and firefighting foam. They contain many desirable properties, such as their ability to lower the surface tension of water significantly (hence their designation as a surfactant). This can be seen in their use in chrome plating, where PFAS are used to prevent the evaporation of hexavalent chromium(VI) vapor. Due to their desirable properties, they are used widely in many industries.
However, they are incredibly dangerous. They have been linked with causing immunotoxicity, thyroid disease, liver disease and cancer, lipid and insulin regulation, kidney disease, uric acid, kidney cancer, and adverse reproductive outcomes. Moreover, they have been detected in blood samples of people throughout the world. PFAS also have bioaccumulative properties in water, meaning they can reach relatively high concentrations in water we as humans consume.
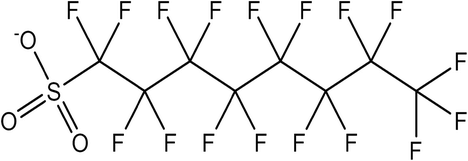
PFAS chemicals consist of a hydrophobic, nonpolar fluorocarbon chain of varying length and a hydrophilic, polar head group such as carboxylate or sulfonate. Below is an image of PFOS, one of the most widely used PFAS chemicals. They have a naming convention as follows: PFXY, where PF stands for perfluoro, X is the carbon chain using hydrocarbon naming convention (B = 4 carbons for butane, Pe = 5 carbons for pentane), and Y is the functional group (A for carboxylate and S for sulfonate). PFAS can be further categorized by whether they are long or short-chain, depending on, you guessed it, the length of their fluorocarbon chain. Short-chain carboxylic PFAS (PFAS with a carboxylate head, also known as PFCAs), have 7 or fewer carbons while short-chain sulfonic PFAS (PFAS with a sulfonate head, also known as PFSAs), have 5 or fewer carbons. Due to the differences in chain length, short and long-chain PFAS have significantly different properties.
PFAS chemicals were not always seen as dangerous. In the 70s and 80s, many chemical companies denied or downplayed the effects of PFAS in humans, and not much research was performed on their impacts on humans until recently (the last 20+ years). The first regulation for PFAS was the 2004 Stockholm Convention on Persistent Organic Pollutants, which resulted in trade restrictions for the two main “legacy” PFAS known as PFOA and PFOS. Most recently, the EPA has placed a maximum concentration level for PFOA and PFOS at 4 ppt in drinking water. There was also a landmark court case where 3M, the company that produces PFAS chemicals, offered $10 billion to settle claims of PFAS contamination in 12,000 US water systems. Since the 2000s, companies that have produced PFAS have switched to using short-chain PFAS instead of long-chain PFAS to skirt these regulations, which have been mainly placed on long-chain PFAS.
Even though long-chain PFAS such as PFOA and PFOS have been mostly phased out of the industry at this point, companies like 3M and DuPont have switched to using short-chain PFAS such as PFBA, PFBS, and GenX (6 carbons). Most of the research regarding PFAS has been performed on long-chain PFAS, which is why research on short-chain PFAS is both novel and important.
PFOS Molecule:
There are key differences in properties between long and short-chain PFAS. Since long-chain PFAS chemicals have a longer nonpolar fluorocarbon chain, making the overall molecule less polar than short-chain. This makes them less soluble in water than short-chain, and they are also larger with a greater surface area than short-chain PFAS.
With the ever-increasing need for industrial products containing PFAS, there is an ever-increasing need for technologies that can remove PFAS from water. There have been shown to be two main methods for PFAS removal: adsorption and membrane separation.
Adsorption (not to be misconstrued as absorption) is a key method for PFAS removal from water, and the predominant forces that govern it are electrostatic and hydrophobic interactions between PFAS molecules and adsorbent material. The positively charged adsorbent material and the negatively charged PFAS molecule attract each other, leading to the PFAS being attached to the adsorbent material as opposed to remaining in water. Due to the increased water solubility and smaller size, short-chain PFAS have been shown to be less efficiently adsorbed than long-chain PFAS for most technologies. For example, the most commonly used adsorbent technology for PFAS, granulated activated carbon, achieves a removal rate in the high 90s for long-chain PFAS but a mere 40% removal rate for short-chain PFAS.
For this reason, there are a lot of new technologies that have been tested with their main removal technique being PFAS adsorption. However, many of them struggle in non-lab environments, such as wastewater. Wastewater naturally contains organic matter, which can compete with PFAS for adsorbent sites, reducing the removal rate. Due to the inefficiencies that can come from external factors such as organic matter and presence of anions, techniques that ONLY use adsorption as their primary removal mechanism are not as effective as the second technology mentioned, membrane separation.
Membranes have been shown to be effective in their removal of PFAS because they use separation based on pore size. Water is allowed through, but the PFAS chemicals get blocked by the membranes, which can have pores smaller than 1 nm. Recently, there has been a lot of research looking into membranes as separators of PFAS, specifically short-chain PFAS. The only potential downsides to some membranes is price. For example, reverse osmosis membranes, which have been shown to be effective in PFAS removal, are incredibly expensive to both install and run due to the amount of energy required. For this reason, there are researchers looking into hybrid membranes that use adsorption and membrane separation to remove PFAS, which can potentially be a lot less expensive to install and use.
There has been a push by legislators to declare PFAS as a class of dangerous chemicals which should be regulated. As of right now, there are major regulations on only four types of PFAS. However, as a new generation of PFAS chemicals emerge, as well as their numerous precursor compounds, the amount of PFAS chemicals has only been growing. Currently, it is estimated that there are over 10,000 PFAS chemicals out there, with more on the way. Legislators argue that without treating PFAS as a class, companies can skirt regulations by just producing new types of PFAS that have not been researched before and declare them safe.
What do you think about this? Should all PFAS be regulated the same? Should there be more regulations for PFAS?

Leave a Reply